
Above and beyond RF
Experience a modern device that delivers complete and consistent depth of coverage, without using RF energy only to ablate endometrial tissue.

Experience a modern device that delivers complete and consistent depth of coverage, without using RF energy only to ablate endometrial tissue.
Dynamic
Plasma dynamically targets least ablated tissue
Precise
The right dose of power for each patient
Effective
Best-in-class clinical results
For Indications, Safety, and Warnings click here.
During the ablation cycle, plasma seeks out the least ablated tissue or tissue that offers the lowest resistance–or does not impede–plasma energy. For complete coverage, heated intrauterine cavity fluids flow throughout the sealed cavity, ablating tissue not in direct contact with the array.
As tissue is desiccated, resistance–or impedance–increases in that area. In response, the plasma changes course and seeks the least ablated tissue or tissue that offers the lowest resistance.





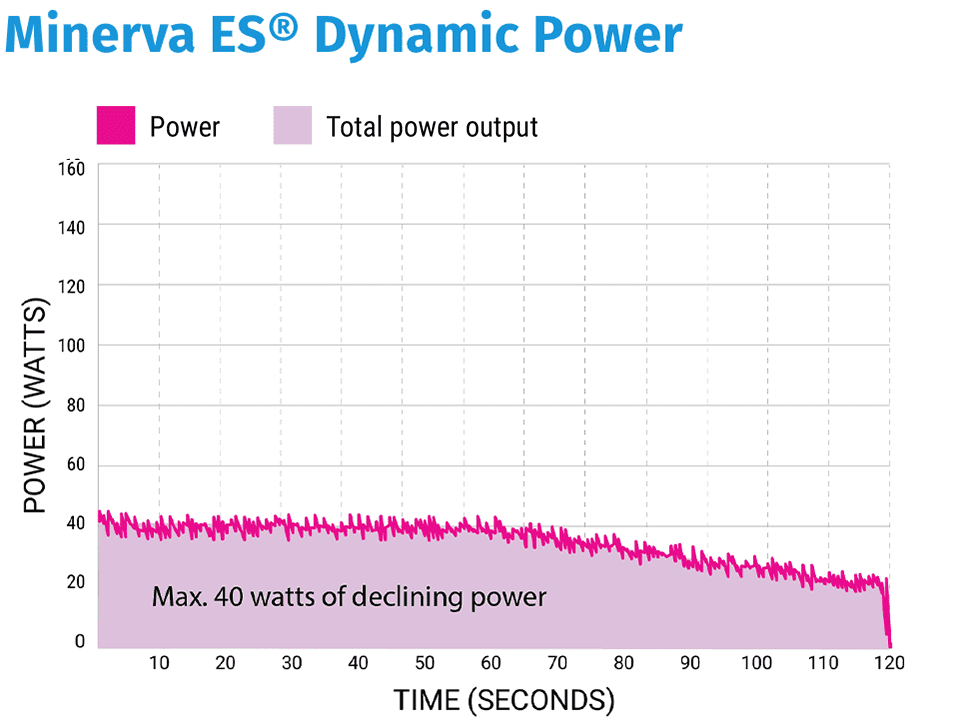
PlasmaSense Technology continuously monitors the uterine cavity (50X per second) to dynamically direct plasma to the least ablated tissue. In response, the power output automatically decreases in real-time as the tissue impedance increases.
Starting with just 40 watts of declining power prevents the uterine cavity from being overwhelmed with energy, which may result in an early impedance shut off with a classic RF ablation.
In 120 seconds, a complete and consistent depth of ablation is achieved.
This is why Minerva ES delivers the best results.
The videos below show actual Minerva ES ablation data as collected in real time by a graphical data software program for comprehensive analysis of data. This data is available to your team during treatments.
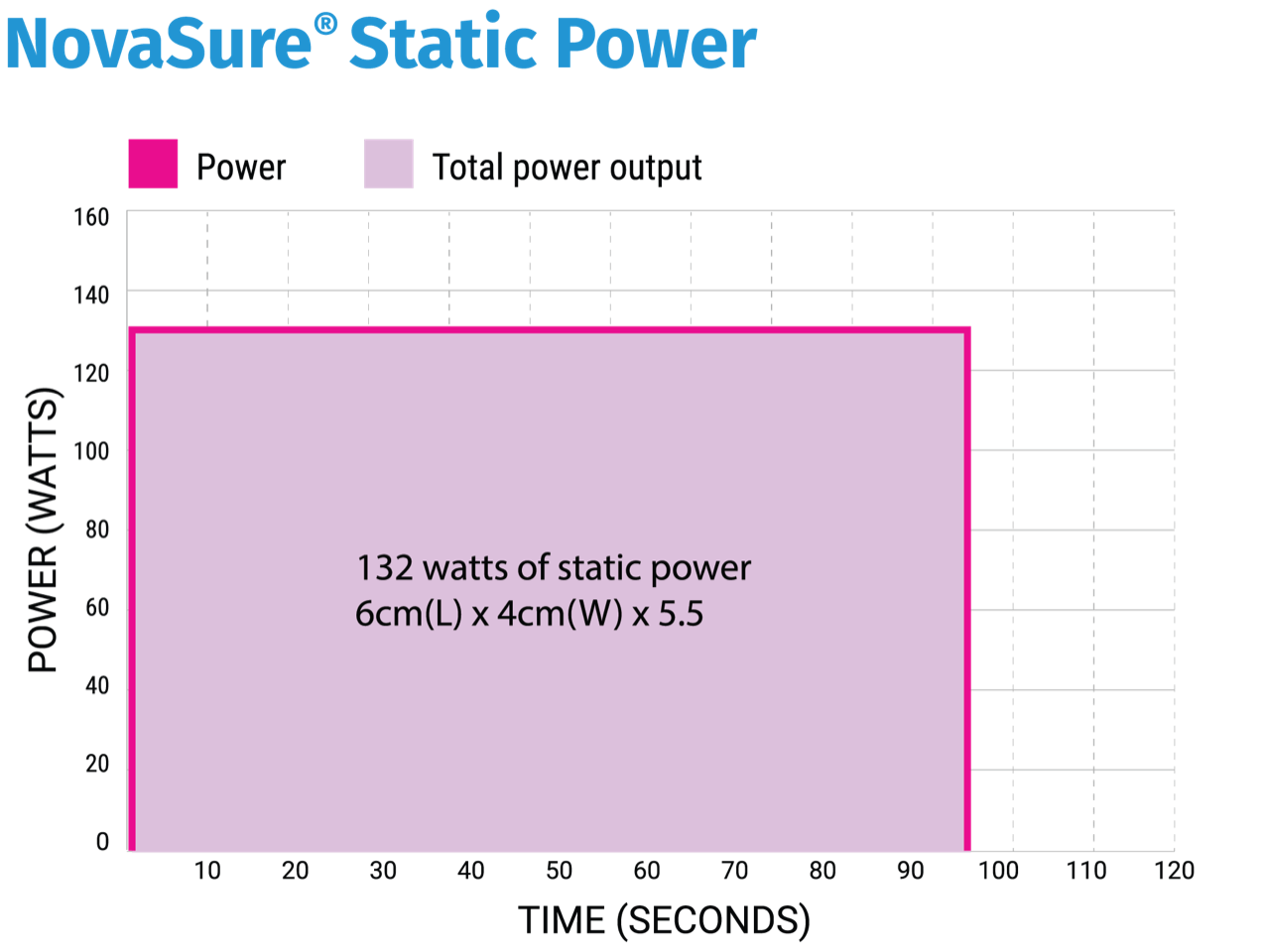
The red line shows the power dynamically responding to an increase in ablated surface area impedance by decreasing accordingly. The yellow line represents increasing impedance, and together they show the power output declining as the area of ablated tissue increases. The cavity receives the minimum amount of power required to ablate from start to finish–far less than the classic RF ablation.
1. FDA Approved Labeling: NovaSure Impedance Controlled Endometrial Ablation System [Instructions For Use and Controller Operator’s Manual]. Marlborough, MA: Hologic, Inc.; 2014.
2. FDA Approved Labeling: Minerva Endometrial Ablation System [Operator’s Manual]. Santa Clara, CA: Minerva Surgical Inc.; L0120, 2020.

For your convenience we’ve selected a series of our most viewed videos. Please visit the Resource Center for access to our complete video library.
Device details. Reimbursement guides. Clinical studies, social media posts, expert opinions and patient outreach programs. Everything you need in one place.
Every patient will receive a 120 second treatment cycle, however the amount of energy delivered during the 120 seconds will vary from patient to patient depending on uterine cavity size and thickness of the endometrium. Each ablation starts with a maximum of 40W of power and declines over the course of the ablation as the impedance within the cavity increases.
Minerva ES does not rely solely on direct tissue contact to achieve optimal ablation. Three simultaneous and complementary methods of tissue ablation are being employed by Minerva, providing a truly global ablation.
Minerva ES is the only ablation system that modulates the power level over the course of the ablation cycle. The controller adjusts power in real time by assessing the changes in the state of the uterine cavity during the ablation process, with measurements being taken and adjustments made 50 times per second. During the ablation process, the area of unablated tissue decreases, and power starts dropping accordingly, with plasma being redirected. Thus focusing ONLY on the least ablated endometrium for a uniform surface area coverage and depth of ablation.
Plasma is another term for ionized gas, in this case argon. The argon gas inside the membrane is ionized by the RF energy delivered to the handpiece from the Minerva Controller.
The cervix should be dilated. An 8mm Hegar dilator is included in the device packaging. The dilator has graduated length markings to ensure accurate measurement of the cervix.
Do not turn off the Controller. Most error codes may be resolved via troubleshooting or replacing the device. Then the Controller will proceed through the safety checks and resume and ablate for the remaining treatment time. In rare instances, a hard fault may be encountered that would require cycling off power to the Controller. Consult the Operator’s Manual or call Minerva for more information.
A maximum of 2 times. If more inflations are needed, reassess to make sure the device is properly positioned in the uterine cavity and rule out uterine perforation.
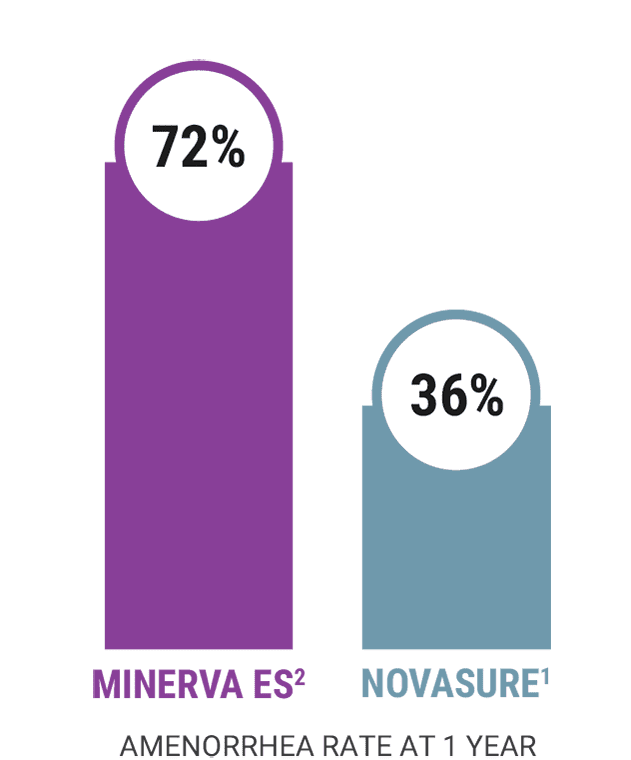
In a controlled clinical study conducted for a FDA approval, Minerva ES achieves an Amenorrhea rate of 72%, or 7 out of 10 women.
Minerva has a low hysterectomy rate, with only 1 out of every 100 patients requiring hysterectomy at 3 years, compared to 6 out of every 100 patients for the closest competitor.
According to the FDA, hysteroscopy is optional when performing endometrial ablation.
The argon cartridge will last for approximately 7 treatments. A CO2 cartridge will last for approximately 25 treatments.
No. According to the product labeling, the use of Minerva ES, and all other second-generation endometrial ablation technologies, is contraindicated in a patient with any anatomic condition that could lead to weakening of the myometrium. Please consult instructions for use for complete indications, contraindications, warnings and precautions.
The Minerva treatment should not be performed concomitantly with the placement of the Essure device. The safety and effectiveness of the Minerva treatment has not been evaluated in patients with the Essure device.