Engineering the complexity out of AUB care
Be there for her with exquisitely simple, device-enabled, non-hormonal solutions that preserve her uterus and provide safe and effective diagnosis and treatment of AUB in any setting. To learn more about the prevalence of AUB and how you can see and treat more women, visit the Resource Center.
This synopsis is a great place to start.
Synopsis: Treating AUB in Your Practice
(5-minute read)

OR Staff
Like second nature, the first time
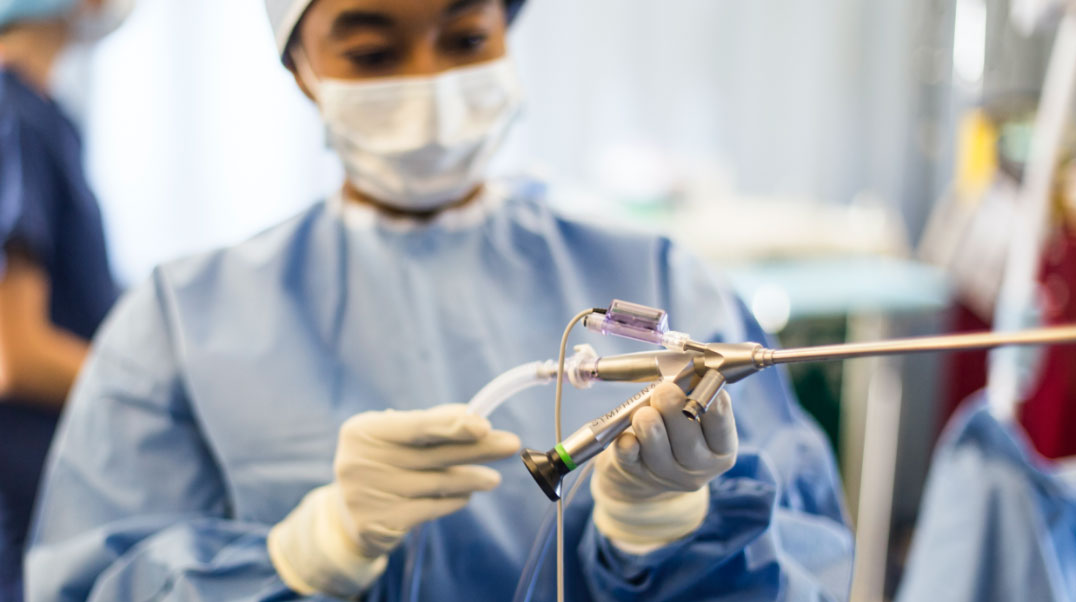
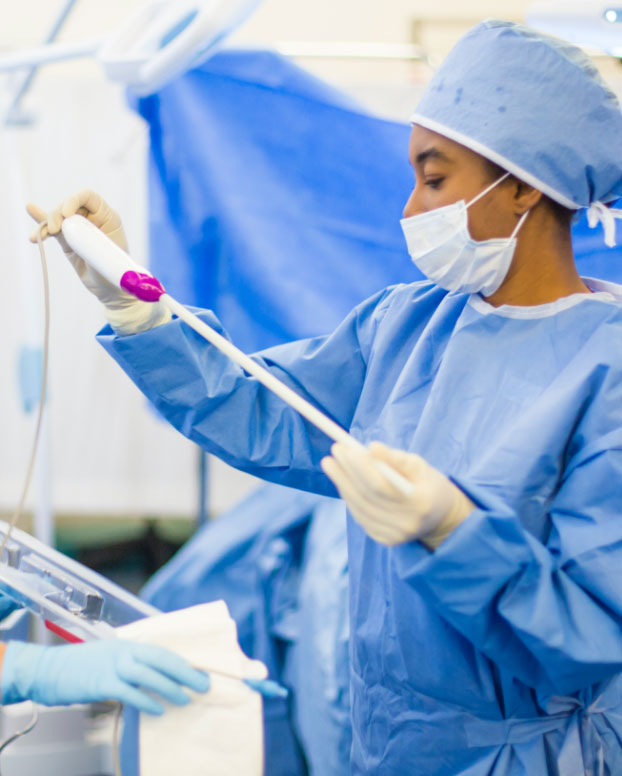

The number of details you juggle under stressful circumstances is staggering. So we invest in continuous improvement of our entire suite of devices, with an emphasis on simplifying setup so it is intuitive and fast. Plus live and on-demand technical support so each case is smooth from start to finish.
Visit the OR Staff Resource Center for quick start guides and set up videos for each device.
Practices
You ask. We deliver.
(In an instant)
It seems that each day involves answering a thousand questions from a million people, all while making patients feel safe and cared for. So we built a virtual assistant for you, ready with every resource you need to effectively treat your AUB patients and optimize your practice performance.
Here’s a kit to get you started. It’s everything you need to support the prioritization of treating AUB in your practice.
Treating AUB in Your Practice: The Kit

Hospitals
Together, we can commit to
Provide women relief from AUB
Your impact on a community is immeasurable. You serve as an anchor, ensuring access to care and a safe place to turn for those with health challenges.
AUB is a condition widely experienced in your community. Up to one third of women suffer with AUB1.
This debilitating condition is under-reported, under-treated and normalized.
A meta-analysis of US and European studies estimated that women with AUB have poor health related quality of life, below the 25th percentile of that of the general population2.
The annual direct and indirect costs of AUB were estimated to be upwards of $1 and $12 billion, respectively2.
Now, with an effective, safe and complete Intrauterine Care Kit, your teams have simple, cost-effective tools to treat the root causes of AUB in all settings. And one company dedicated to supporting you as we advocate together for exceptional and modern uterine care for women.
Treating AUB in Your Community Starts With You.
